
Published on the 8th March 2017 by ANSTO Staff
ANSTO has contributed to experiments using multiple techniques, which have determined, for the first time, the location of interstitial oxygen atoms in a new apatite-type solid electrolyte.
The findings, which have been published in Advanced Functional Materials, are significant because they have implication for the broad class of functional oxides.
Apatite-type materials have shown high oxide-ion conductivity in the intermediate temperature range (500-650 °C) for solid-oxide fuel cells and have applications in oxygen sensors and pumps, oxygen-separation membranes, and as catalysts.
The electrolyte compound Bi2La8[(GeO4)6]O3 was synthesised by lead author Matthew Tate and co-author Dr Ivana Evans from Durham University in the UK.
 |
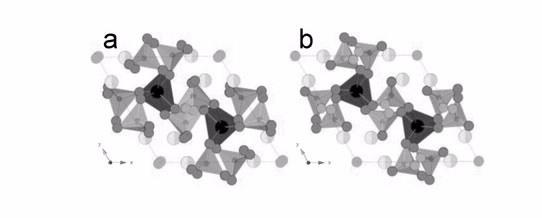
| a) The triclinic room-temperature structure of Bi2La8[(GeO4)6]O3 viewed down the c-axis. b) The hexagonal structure of Bi2La8[(GeO4)6]O3 at 830 °C viewed down the c-axis. [A|] (La) = black, [A||] (La/Bi) = light gray/lighter gray, Ge = small dark gray, O1–O3 = dark gray, O4 = mid gray, and O5 (interstitial) = gray. |
X-ray diffraction on the powder-diffraction beamline at the Australian Synchrotron by co-author Dr Helen Brand and neutron powder diffraction on the ECHIDNA instrument at the Australian Centre for Neutron Scattering (ACNS) assisted by co-author Dr Max Avdeev determined the average crystal structure of Bi2La8[(GeO4)6]O3 at room temperature.
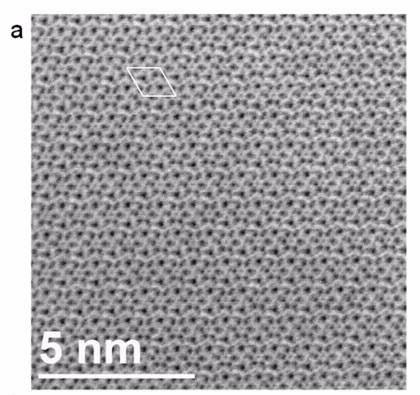 |
| Experimental ABF image for the [001] orientation of Bi2La8[(GeO4)6]O3 Image: University of South Carolina |
To complement diffraction methods, authors Dr Thomas Vogt and Dr Douglas Blom used annular bright-field scanning electron transmission microscopy (ABF STEM) and frozen-phonon simulations at the University of South Carolina in the US to clear up some ambiguity about the location of the oxide ions over all possible sites.
The microscopy, supported by simulations, is believed to be the first direct imaging of interstitial oxygen atoms which add only eight electrons to the 1022 electrons per formula unit of the material.
Structural determination
“All of the apatite-type oxide-ion materials have roughly the same structure. They have a framework with channels and the oxygen atoms run in the channels—providing conductivity,” said Max Avdeev.
“The problem has been that the oxygen atoms are very delocalised and it is very difficult to determine where they are.
Now, through the use of complementary techniques, there is strong evidence of their position in the unit cell,” said Avdeev.
Matthew Tate spent two years at ANSTO working on his PhD under the joint supervision of Dr Garry McIntyre, Research Leader at the ACNS, and Dr Ivana Evans from Durham University.
Both Tom Vogt and Ivana Evans, experts in solid-state chemistry and the application of X-ray and neutron diffraction, undertook sabbaticals at ANSTO to conduct a number of experiments on the ECHIDNA instrument.
To characterize fully the crystal structure, other techniques used in the experiments included AC impedance spectroscopy and second-harmonic-generation measurements.
Neutron diffraction was also undertaken at high temperature to investigate a phase transition in the material at 830° C, in which the triclinic structure changes to a reversible hexagonal structure.